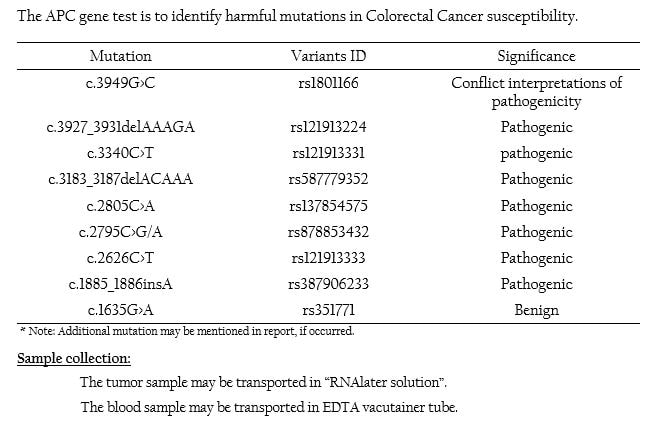
APC Mutation Panel
APC Gene overview:
- Adenomatous polyposis coli (APC) is a tumour-suppressor gene whose protein functions as a key antagonist of the WNT signaling pathway by binding and regulating β-catenin due to which it is also considered as the ‘gatekeeper’ gene[1]
- Inactivation of APC results as an early event for the development of Colorectal Cancer (CRC) and may play a pivotal role in triggering the adenoma-carcinoma pathway.
- Also involves in promoting the process of tumourogenesis through loss of cell-cell adhesion[1&2].
- Overexpression of APC stimulates G1 phase arrest in colorectal cancer and when APC mutates, it fails its role of maintaining the G1/S checkpoint, thereby contributing to aberrant cell proliferation[3].
- APC is the most frequently mutated gene and also a known driver gene in case of colorectal cancers[1].
- APC is regarded as CRC tumour suppressor gene and it is found to be dysregulated at both germline and somatic mutations[4]
- The exon 15 contains nearly 75% of the coding region and it appears as the most common region for both germline and somatic mutations of APC[5].
- Germline mutations in APC gene is considered as the major hereditary predisposition event leading to Familial Adenomatous Polyposis (FAP)[6].
- All mutations lead to truncation of the APC protein either by nonsense (30%) or by frameshift mutations (68%). The majority of mutations occur within the first half of the coding sequence.
- Somatic mutations are found in more than 80% of the sporadic colorectal tumours and 30-40% of the CRC cases are due to loss of heterozygosity[7&8].
- Carriers of germline APC mutations have virtually 100% chance of developing CRC by age 40, and most sporadic cancers have somatic mutations or deletions in this gene[9].
- Somatic mutations have been associated with malignant transformation of adenomas.
- About 60% of the APC mutations lie in the Mutation Cluster Region (MCR)[10].
- With respect to therapeutic implications, CRC cells harboring mutant APC may be more vulnerable to DNA damaging chemotherapeutic agents, such as oxaliplatin and 5-fluorouracil[11].
- There is enough evidence to support the fact that APC usually co-occurs with KRAS or P53 mutations or both in CRC development[12&13]
- Fearon ER.(2011). Annu. Rev. Pathol, 6:479-507.
- Bienz M. and Hamada F. (2004). Curr Opin Cell Biol, 16(5):528–535.
- Heinen CD, et al., (2002). Gastroenterology, 123(3):751–763.
- Fodde R, et al., (2001). Nat Rev Cancer, 1(1):55-67.
- Beroud C and Soussi T. (1996). Nucleic Acids Res, 24(1):121–124.
- Kinzler KW and Vogelstein B.(1996). Cell, 87(2):159–170.
- Fearnhead NS, et al., (2001). Hum Mol Genet, 10(7):721–733.
- Rowan AJ, et al.. (2000). Proc Natl Acad Sci USA, 97(7):3352–3357.
- Triantafillidis JK, et al, (2009). Anticancer Res, 29:2727-2737.
- Miyoshi Y, et al., (1992). Hum Mol Genet, 1(4):229–233.
- Lu Z and Jerry WS. (2017). J Natl Cancer Inst, 109(8).
- Markowitz SD and Bertagnolli MM. (2009). N. Engl. J. Med, 361:2449–2460.
- Jones S. et al., (2008). Proc. Natl Acad. Sci. USA, 105:4283–4288.