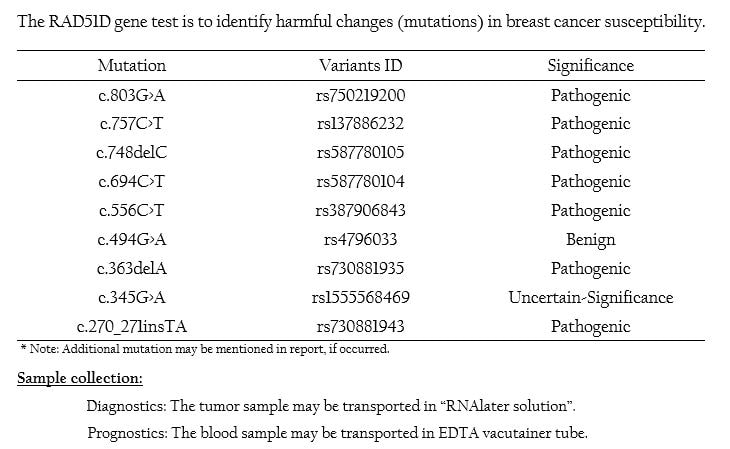
RAD51D Mutation Panel
RAD51D overview:
- RAD51D is a member of the RAD51 protein family and is involved in DNA repair mechanism by homologous recombination (HR).
- This is a DNA recombinase which is basically involved in the homologous recombination occurring in eukaryotic cells[1].
- HR mechanism is essential to maintain genome integrity in a cell, and HR loss of function has been strongly linked to breast cancer.
- It is possible that carcinomas arising in patients carrying RAD51D mutations will be sensitive to chemotherapeutic agents that target this pathway, such as cisplatin and the poly (ADP-ribose) polymerase (PARP) inhibitor olaparib.
- Mutations in RAD51 are also one of the major factors causing high risk of cancer occurrence in breast and ovary[2&3].
- Missense mutation of RAD51D was identified in individuals presenting Breast and Ovarian cancer.
- RAD51D is mutated in women affected by familial ovarian cancer with or without breast cancer[4].
- RAD51D Mutation will also be of potential value to female relatives, on average, at an approximately six-fold increased risk of ovarian cancer.
- Women who carry RAD51D pathogenic mutations are 4 to 12 times more likely to develop ovarian cancer than women in the general population.
- The lifetime risk of RAD51D mutation in ovarian cancer patients is estimated to be approximately 10% by the age of 80[5].
- RAD51D mutation status is important for the female relatives of affected patients, as this knowledge may allow them to make informed decisions about preventive options to mitigate their elevated risk for disease.
- The ovarian cancer relative risk for RAD51D mutation carriers was estimated to be 6.30%.
- The cells which are RAD51D-deficient are highly sensitive to PARP (Poly-ADP-Ribose Polymerase) inhibitors which are similar to BRCA1 and BRCA2 deficient cells.
- Though the occurrence of RAD51D is a rare phenomenon, determining its mutation status is important for taking precautionary measures.
- The patients having RAD51D mutations may also be sensitive to the chemotherapeutic agents such as cis-platin etc., which targets the RAD51D pathway[4&6].
- When a person is tested with RAD51D mutation, the physician highly recommends regular monitoring to check the development of the cancer.
- There are research evidences that shows that monitoring plan improves the survival of individual with a RAD51D mutation who did not have any signs and symptoms of cancer.
- Monthly breast self-examination, starting at the age of 18.
- Clinical breast examination twice a year, annual breast MRI beginning at age 20 to 25.
- Masson JY, et al., (2001). Genes Dev 15:3296-307.
- Loveday C, et al., (2012). Nat. Genet. 44, 475–476
- Pelttari, LM, et al., (2011). Hum. Mol. Genet. 20, 3278–3288
- Loveday C, et al., (2011). Nat Genet 43(9): 879 - 882.
- Osher DJ, et al.,(2012). British Journal of Cancer, 106(8), 1460-1463.
- Banerjee S, et al., (2010). Nat Rev Clin Oncol 7(9): 508 – 519.